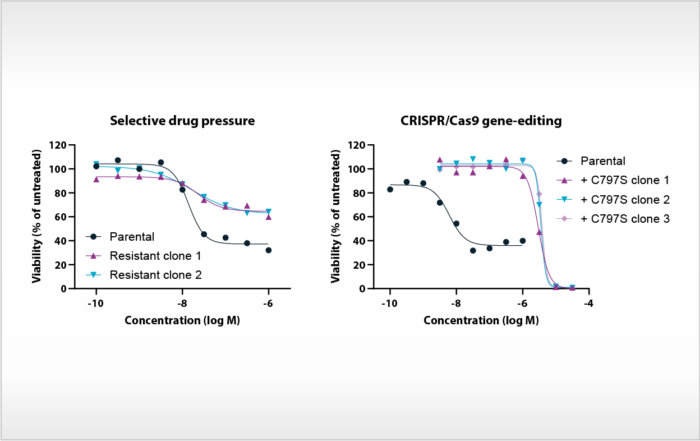
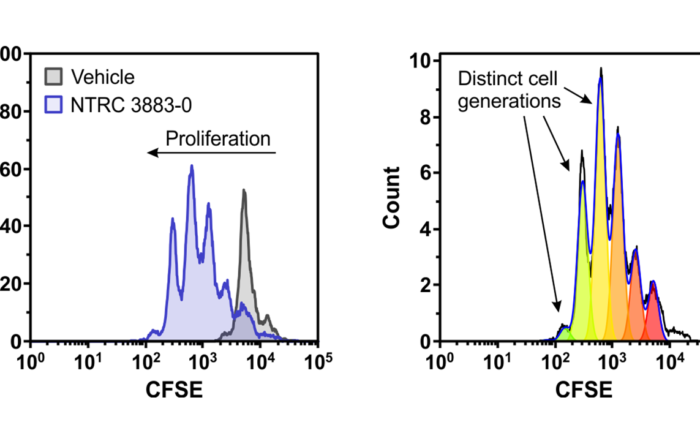
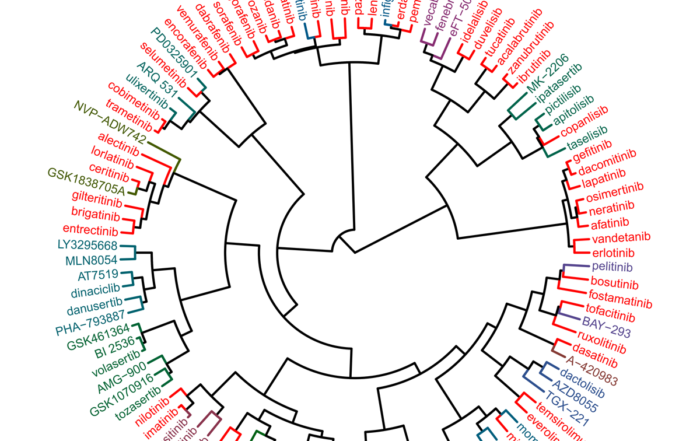
Compound profiling on EGFR mutants and osimertinib-resistant cell lines
Mutations in the epidermal growth factor receptor (EGFR) are the most common oncogenic drivers in non-small cell lung cancer (NSCLC), but many patients treated with EGFR kinase inhibitors develop drug resistance. At Oncolines, [...]
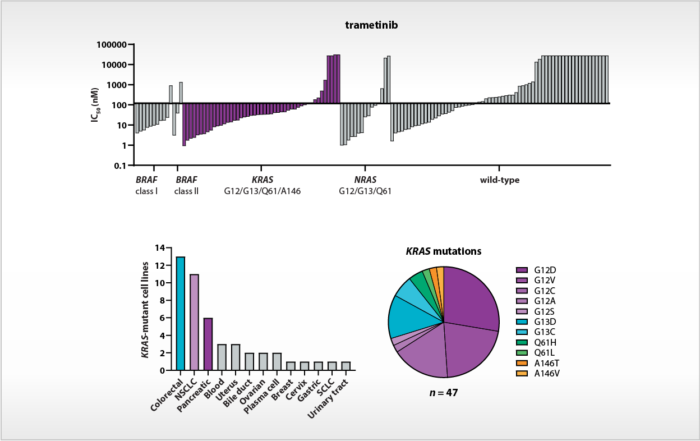
Cancer cell panel profiling of MAPK pathway inhibitors
The MAPK signal transduction pathway plays a key role in tumor progression. Mutations in the corresponding driver genes, such as KRAS and BRAF, are oncogenic drivers in several malignancies. Tumors driven by V600-mutated [...]
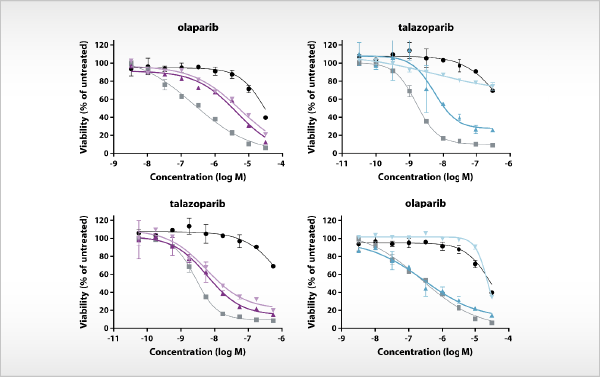
Compound profiling in PARP inhibitor-resistant cancer cell lines
Drug resistance is a major problem in the treatment of cancer. Many aspects of clinical drug resistance can be studied in drug-resistant cell lines generated in vitro. At Oncolines, there are more than [...]
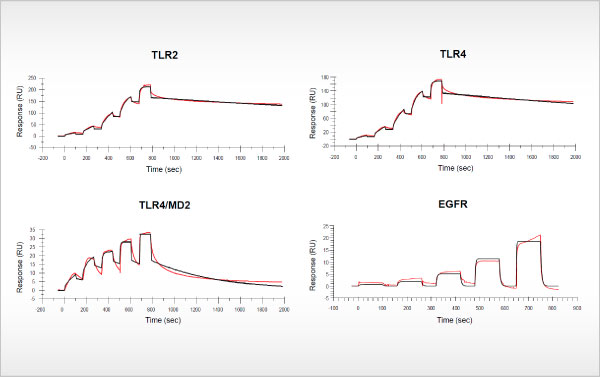
Oncolines contributes to publication in Mucosal Immunology
Biacore studies prove binding of bacterial lysate OM-85 to Toll-like receptors OM-85, also known as Broncho-Vaxom®, is an extract of more than 21 different respiratory pathogenic bacterial strains that is indicated for the prevention [...]
Oncolines contributes to publication inNature Communications
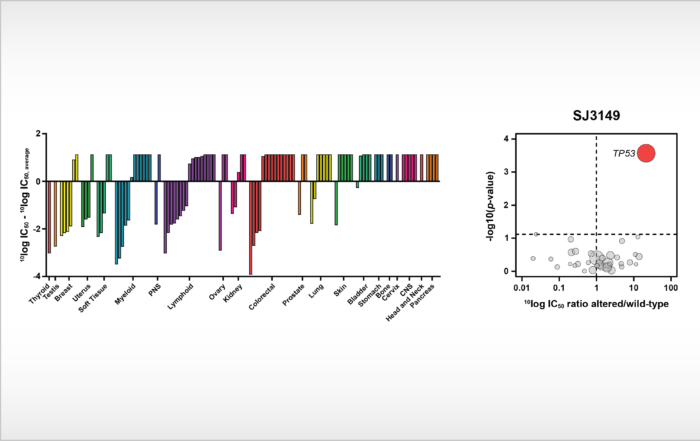
Identification of first-in-class, potent and selective CK1α degrader In a new study, which was published today in Nature Communications, investigators from St. Jude Children’s Research Hospital describe the discovery and characterization of a [...]
New bioinformatics service
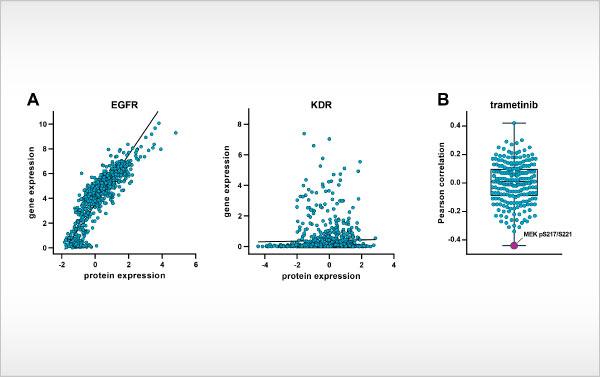
Oncolines launches ProteomicsProfiler Figure (A) Scatterplots of basal gene and protein expression levels for EGFR (left, strongly correlated) and KDR (right, not correlated) in cancer cell lines. (B) Boxplot of Pearson correlations [...]
Oncolines presentation at Revvity Symposium Ghent
Biomarkers for Patient Stratification and Target Engagement Dr. Guido Zaman, Managing Director of Oncolines B.V., will present at the Revvity North Symposium Life Science & Applied Genomics 2023, which will take place [...]
Booth #410 – Oncolines at AACR-NCI-EORTC 2023 in Boston
Molecular Targets and Cancer Therapeutics conference Oncolines will present two posters at the Molecular Targets and Cancer Therapeutics conference in Boston next week. Oncolines will also exhibit in the Exhibition Area (Booth [...]
100 years pharmaceutical industry in Oss
Oncolines team featured in ‘Walk of Fame’
To celebrate the past and future of pharma industry in Oss, a Walk of Fame was opened at the Pivot Park, the biopharmaceutical campus of Oss and home of Oncolines. The Walk of Fame showcases [...]
New case study on Oncolines® website
Target identification of hits from a phenotypic screen Phenotypic screens measure a desired biological effect in a disease-relevant cellular context. In contrast to target-based assays, phenotypic screens are agnostic of a defined molecular [...]
New publication in Cancer Chemotherapy and Pharmacology
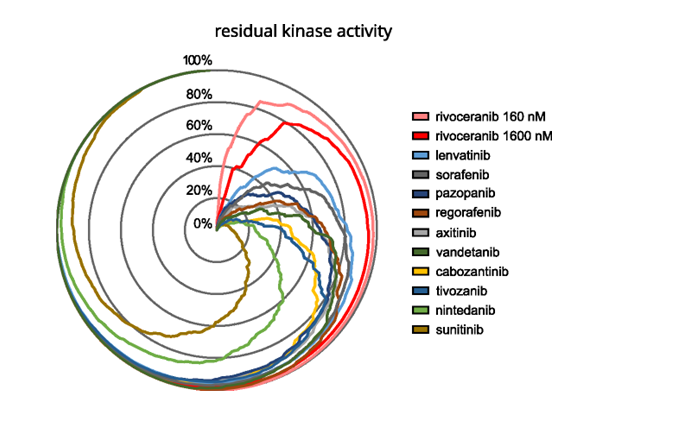
Comparative biochemical kinase profiling of VEGFR2 inhibitors Figure. Radar plot showing residual activity of 270 kinases in the presence of rivoceranib (160 or 1600 nM) or 1000 nM of 10 FDA-approved reference [...]
Oncolines Hosts Webinar: Biomarkers for Patient Stratification & Target Engagement
Despite significant advances made in the treatment of cancer through the past decades, many new therapeutic entities fail in the clinical research phase. To accelerate your drug development process and improve patient treatment, the [...]
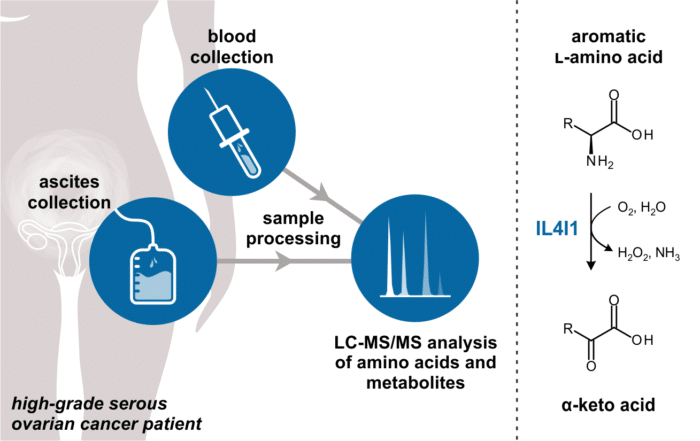
New Oncolines publication in Cancers
IL4I1 in ascites as a marker in high-grade serous ovarian cancer The emergence of immunotherapy has revolutionized cancer treatment for various malignancies. However, immunotherapies have demonstrated only limited efficacy in ovarian cancer, [...]
Oncolines Team Joins Symeres
Symeres acquires Oncolines, further strengthening its drug discovery and biology capabilities Nijmegen, the Netherlands: Symeres, a leading global drug discovery Contract Research Organization (CRO) and Contract Development and Manufacturing Organization (CDMO), [...]
New in Frontiers in Oncology: comparative profiling study of newly approved kinase inhibitors
In the last two decades, nearly 60 small molecule kinase inhibitors have been approved by the FDA for oncology indications. Although the human kinome consists of around 500 kinases, the inhibitors that are [...]
On-demand webinar on cancer genomics
Cancer genomics is key to Oncolines’ cancer cell line profiling services and bioinformatics. In PIVOTTalk, a webinar series hosted by the Pivot Park, Jeffrey Kooijman and Tessa de Bitter describe the history [...]
Oncolines Hosts Webinar: How to Select the Best Platform for Testing Drug Combinations
Many cancer treatments are combination therapies. Combining drugs can improve therapy response, prevent drug resistance, or reduce toxicity. This June, Oncolines hosted a webinar discussing the different approaches available to identify new synergistic drug combinations [...]
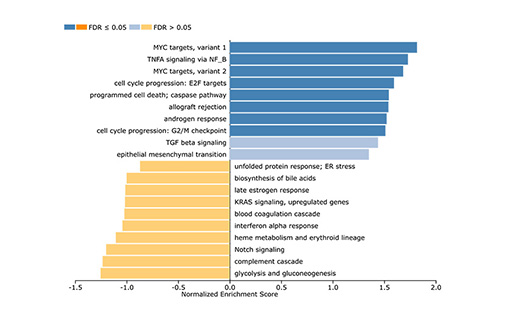
Case study – Gene expression profiling to understand and combat drug resistance
RNA sequencing provides insight into the mechanism of kinase inhibitor resistance Figure: Gene set enrichment analysis indicated Myc target genes were upregulated in the resistant clone Clinical response to [...]
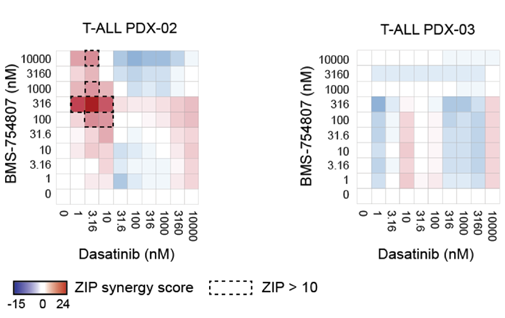
Proposed new combination treatment strategy for pediatric ALL published in Nature Communication
Oncolines contributes to a proposed new combination treatment strategy for pediatric acute lymphoblastic leukemia published in Nature Communication T cell acute lymphoblastic leukemia (T-ALL) is an aggressive cancer arising from aberrant proliferation of T [...]
Oncolines News Flash
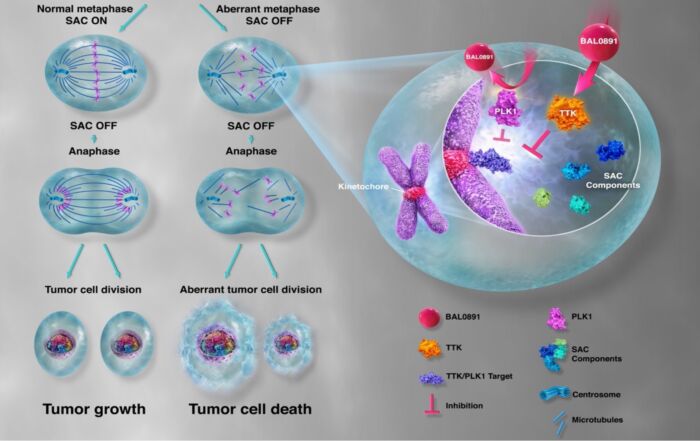
First presentation of novel mitotic checkpoint inhibitor BAL0891 data at ESMO TAT congress 2022 At the virtual Targeted Anticancer Therapy (TAT) congress, which was organized by the European Society of Medical Oncology (ESMO) [...]
Oncolines News Flash
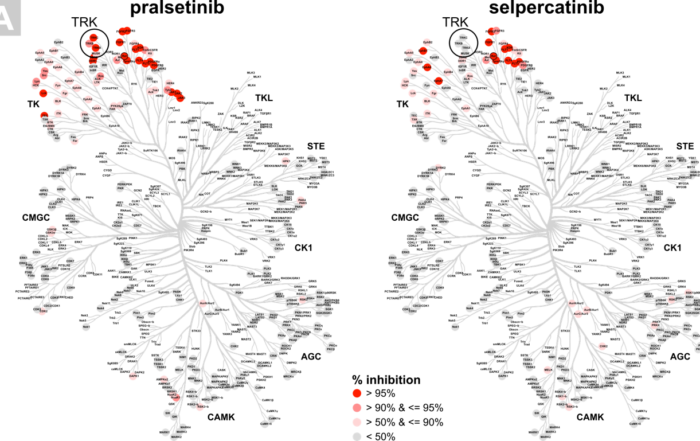
Oncolines to present detailed profiling of recently approved kinase inhibitors at the ESMO TAT Currently approved small molecule kinase inhibitors act only through a limited number of kinase targets. Well-known targets are EGFR, [...]
Oncolines News Flash
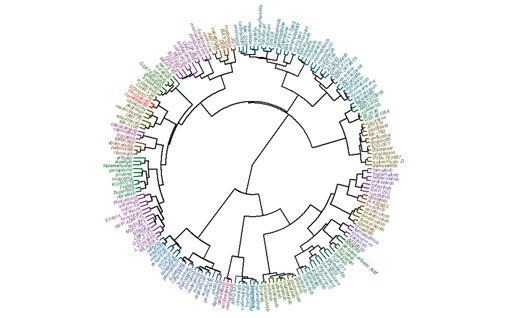
OncolinesProfiler™ Compound Library is approaching the 200 mark. Clustering analysis via OncolinesProfiler™ compares your compound’s IC50 fingerprint against the cellular activity of other anti-cancer therapeutics. The OncolinesProfiler™ compound library started out as a [...]
Case study: Combination matrix to determine Bliss independence synergy scores
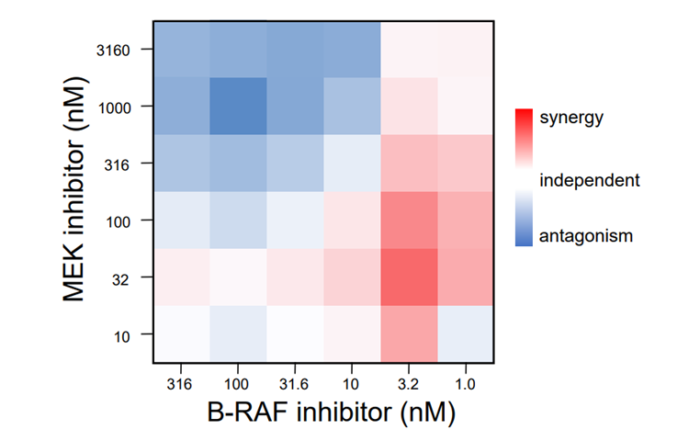
November 19, 2021: A new case study is presented, in which the use of a combination matrix for the determination of synergy is highlighted. The figure shows the combination of the [...]
Oncolines opens a new screening lab
Oncolines is expanding its lab capacity with a new screening lab including cell culture facilities. Oncolines opens a new screening lab Oncolines is expanding its lab [...]
NTRC Precision Medicine Services is now Oncolines
As an independent company, Oncolines will now be singularly focused on offering the best precision medicine services in oncology and cancer immunotherapy for our clients. Our Oncolines™ cancer cell line panels can identify drug [...]
[Webinar] Comparative Cancer Cell Panel Profiling of Recently Approved Kinase Inhibitors
Kinases are the major anticancer drug target class of the 21st century1 with nearly 60 small molecule kinase inhibitors approved for clinical use in the first two decades. Key to the success of kinase inhibitor [...]
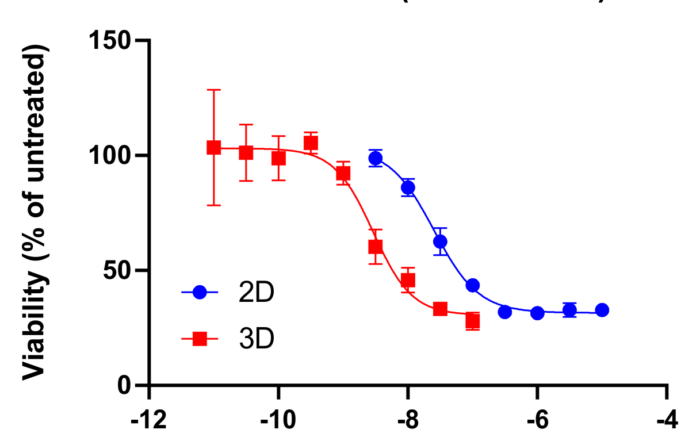
Case study: Altered drug response in 3D spheroids
May 25, 2021: A new case study is presented, in which the use of spheroids for the determination of drug sensitivity is highlighted. The figure shows dose-response curves of the KRAS [...]
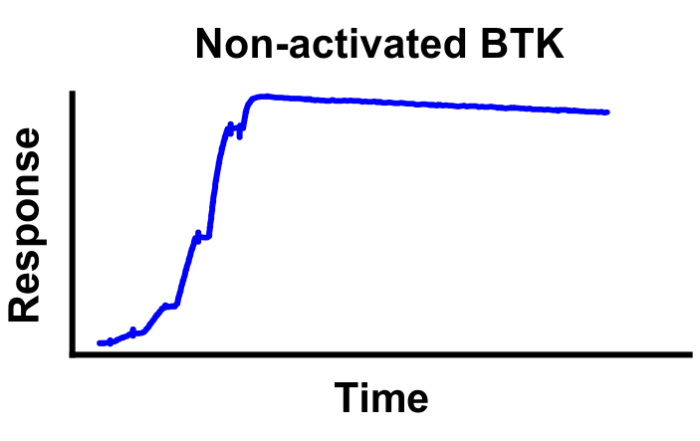
Case study: Phosphorylation status determines kinase inhibitor binding
April 28, 2021: A new case study is presented going in-depth on the biochemical properties of Bruton’s tyrosine kinase (BTK) inhibitors. The figure shows surface plasmon resonance sensorgrams for the binding [...]
Kinase inhibitor profiling and resistance mechanisms presented at the AACR Annual Meeting 2021
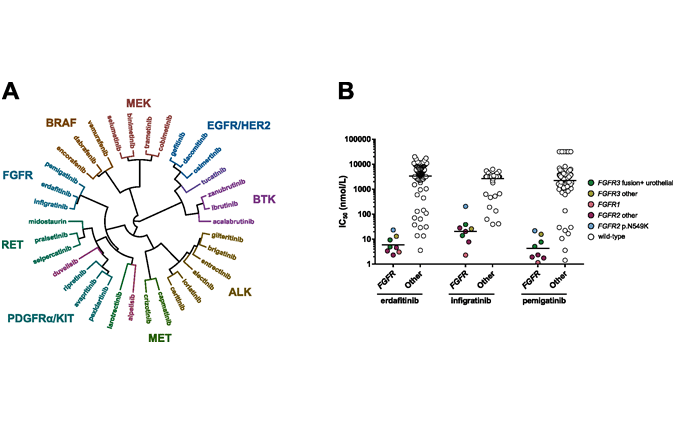
April 8, 2021: NTRC will present two scientific posters at the virtual AACR Annual Meeting 2021. The figure shows results from one of the posters, demonstrating that erdafitinib-resistant AN3 CA cells [...]
[Webinar] How to Overcome Acquired Resistance Against Kinase Inhibitors
Resistance development is all too common in cancer treatment and leads to cancer cells evading targeted therapies. To better understand how these resistance mechanisms work and in order to help develop new therapies, Investigator [...]
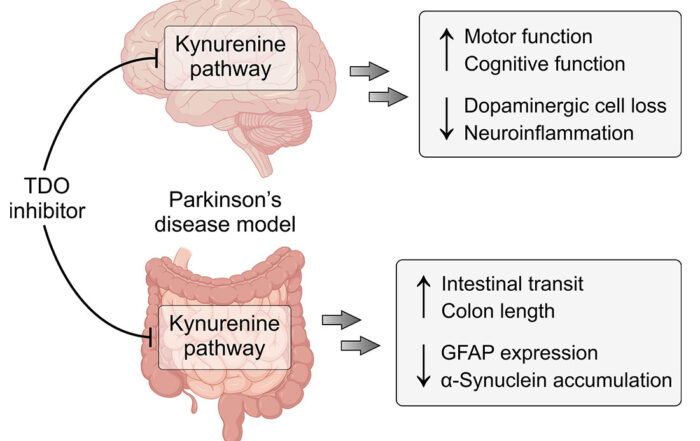
TDO is a novel therapeutic target for Parkinson’s disease
February 22, 2021: A new article on the pharmacological validation of TDO as a target for Parkinson's disease is now online in The FEBS Journal (Perez-Pardo et al., 2021). The figure [...]
Tryptophan-metabolizing enzymes in cancer immunology
January 28, 2021: A new article on the role of tryptophan-metabolizing enzymes in cancer immunology is now online in Frontiers in Immunology (Grobben et al., 2021). The figure shows a co-culture assay of [...]
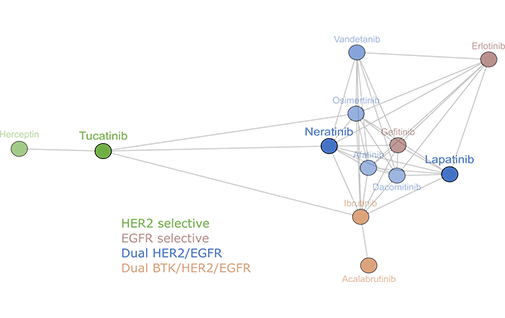
Comparative analysis of drug response and gene profiling of HER2-targeted tyrosine kinase inhibitors
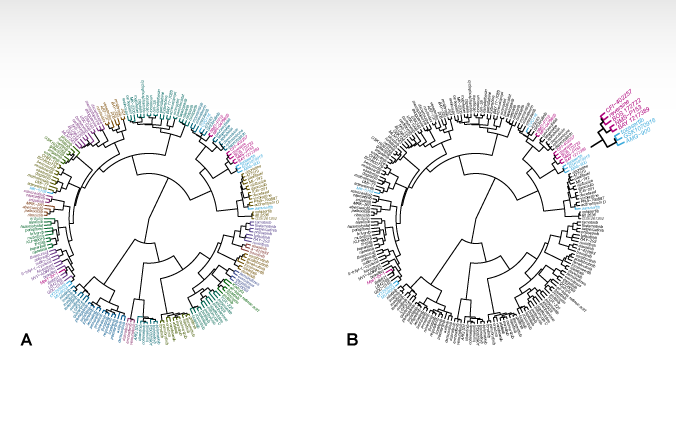
January 21, 2021: A new article on HER2-targeted tyrosine kinase inhibitors is now online in British Journal of Cancer (Conlon et al, 2021). The illustration shows clustering analysis of 12 EGFR and HER2 inhibitors [...]
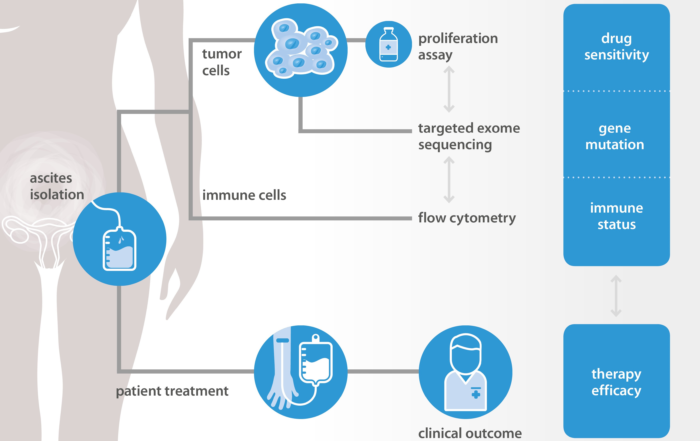
Predictive approach for clinical response to chemotherapy
December 21: A new article on malignant ascites as a model for drug sensitivity testing is now online in Oncotarget (den Ouden et al, 2020). New in Oncotarget: Chemotherapy sensitivity [...]
New website on small molecules for precision oncology: www.ntrctx.com
December 01: Read about NTRC Therapeutics translational science platforms and drug discovery projects at www.ntrctx.com. New website on small molecules for precision oncology: www.ntrctx.com Oss, December 01, Targeted therapies [...]
Presentations at ASH2020
November 30: Join us at ASH2020 for two oral presentations on December 7th, by Jordy van der Zwet and Valentina Cordo. Combination therapies for T-ALL presented at ASH2020 Oss, [...]
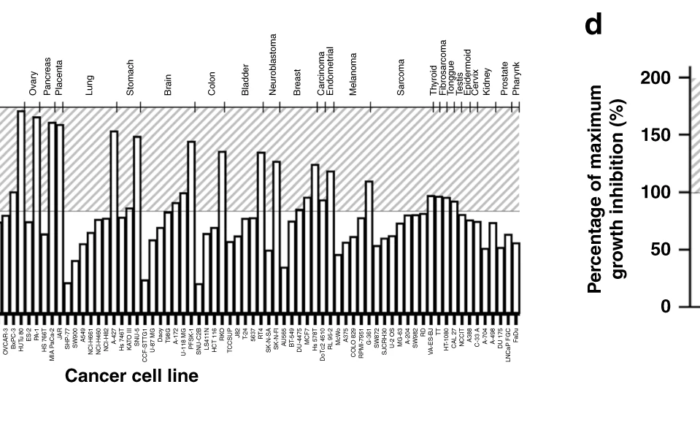
New paper in Nature Communications illustrates the power of cancer cell line profiling
October 29, 2020: Percentage of maximum growth inhibition of various cell lines following 72 h treatment with 1 μM of PCLX-001 using the Oncolines™ cell line screen. Corresponding violin graphs compare the average PCLX-001-mediated growth [...]
Webinar: Using cancer cell panel profiling and bioinformatics to investigate the mechanistic hypothesis for AMG-510
October 26, 2020: Announcement of webinar on cancer cell line profiling and bioinformatics analysis to investigate the mechanistic hypothesis for AMG-510. Webinar - Investigate the Mechanism of AMG-510 via Cancer [...]
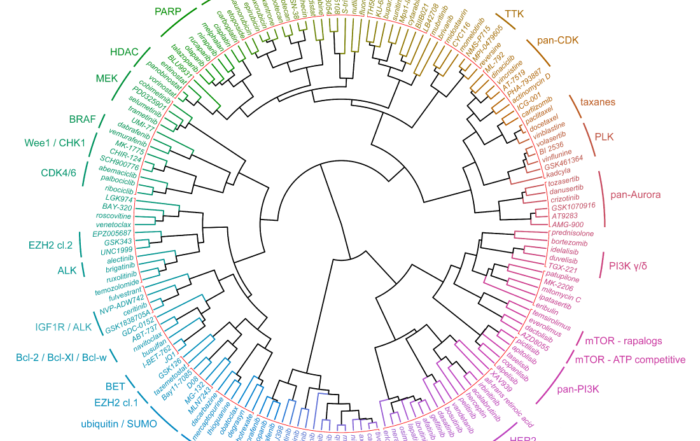
Clustering analysis of recently approved kinase inhibitors presented at Discovery on Target 2020 – Virtual
September 14, 2020: Hierarchical clustering of kinase inhibitors based on their IC50 profiles in the Oncolines™ panel. Names of approved kinase inhibitors are given in red. Clustering analysis of recently [...]
NTRC launches new website: www.residencetimer.com
July 30, 2020: NTRC launches www.residencetimer.com for biochemical assays in drug discovery. Biochemical assays for determination of binding characteristics Oss, July, 30, 2020 – NTRC Precision Medicine Services proudly [...]
NTRC Presents at AACR Virtual Annual Meeting II
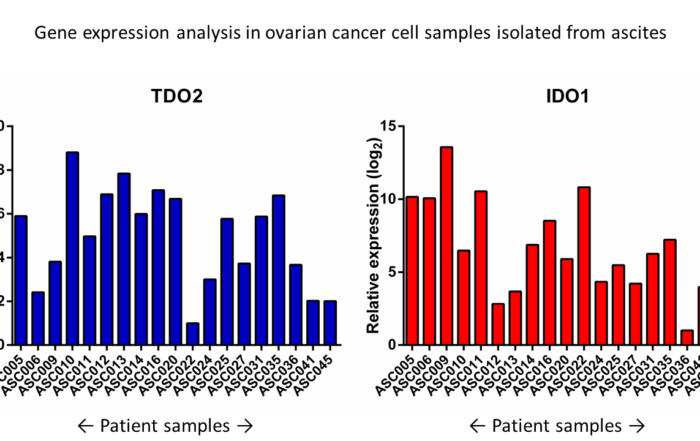
June 23, 2020: NTRC presents the poster #5604, titled 'High TDO and IDO1 expression in ovarian cancer-associated cells isolated from malignant ascites' at the AACR Virtual Annual Meeting II. High [...]
NTRC, Pelago Bioscience and Pangaea Oncology Collaborate in Eurostars Project on a Novel Biomarker Platform for Immuno-Oncology
June 12, 2020: Eurostars collaboration to develop a biomarker platform for Immuno-Oncology, consisting of markers for Target Engagement, Immune Markers, and Genomic Biomarkers. The Eurostars partners are NTRC (The Netherlands), Pelage Bioscience (Sweden), [...]
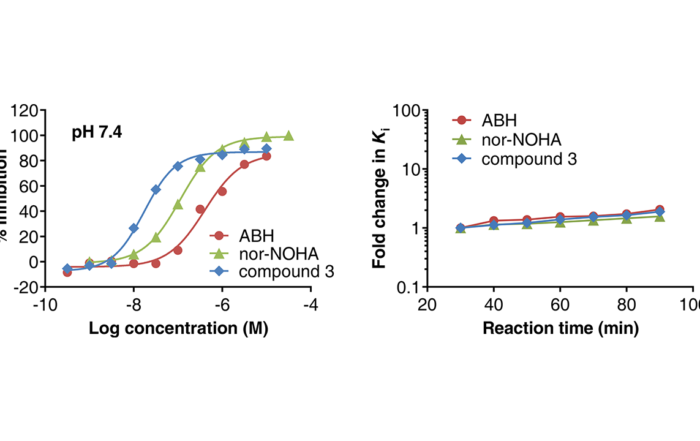
Novel screening assay for Arginase-1 inhibitors published in SLAS Discovery
May 18, 2020: Dose response curves of reference inhibitors in the Arginase Gold™ assay (left) and inhibitory constant (Ki) as function of reaction time (right). Adapted from Grobben et al. (2020). [...]
NTRC Expands its Capacity in Molecular Biology Services
May 04, 2020: NTRC's new laboratory space for Mechanistic Cell Biology. Additional Laboratory Space for Molecular Biology Services Oss, May, 4th, 2020 – Happy to announce that NTRC is [...]
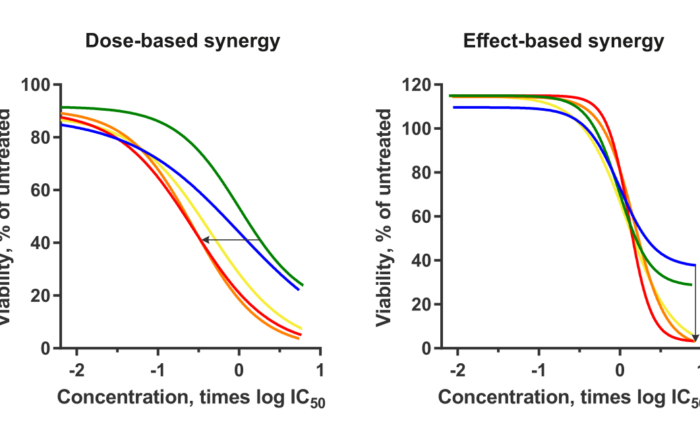
Dose- and Effect-based Drug Combination Methods Merged into One Approach
Left: Dose-based synergy results from a decrease in dose to achieve the same effect, quantified with the CI value. Right: Effect-based synergy results from an increase in the maximum effect, quantified by Bliss [...]
NTRC Participates in Alpe d’HuZes 2020 to Raise Money for Cancer Research
NTRC thanks our sponsors for the cycling event Alpe d’HuZes. Special thanks to our shirt sponsors: Pivot Park, Miltenyi Biotec, Glycostem, Perkin Elmer, Hulshof Kroonen & Groen. NTRC Participates in [...]
Expression of Cancer Immunotherapy Targets in Patient Material
Gene expression of TDO and IDO1 in ovarian cancer cell samples isolated from ascites. Expression of Cancer Immunotherapy Targets in Patient Material Oss, March, 04th, 2020 – ESMO has made [...]
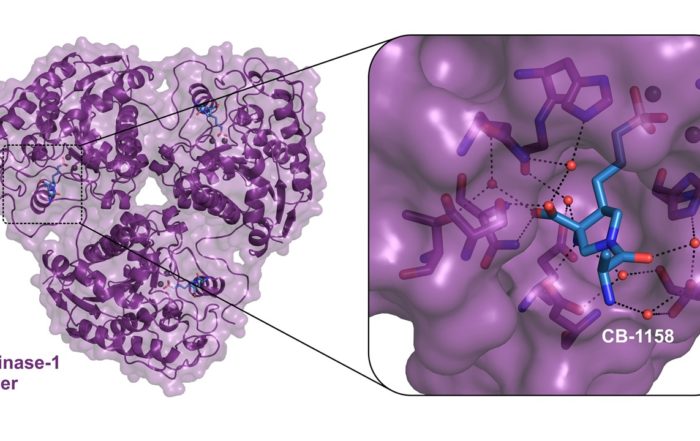
New Structural Insights into Arginase-1, a Target for Cancer Immunotherapy
X-ray protein crystal structure of human Arginase-1 with the small molecule inhibitor CB-1158. New structural insights into Arginase-1, a target for Cancer Immunotherapy Oss, November, 29th, 2019 – NTRC scientists [...]
A Precision Medicine Platform to Predict the Clinical Response to Chemo- and Immunotherapy
At the 2019 ELRIG Drug Discovery Conference 'Looking Back To The Future', NTRC will present on a predictive approach for targeted therapy based on in vitro screening of primary material of ovarian cancer [...]
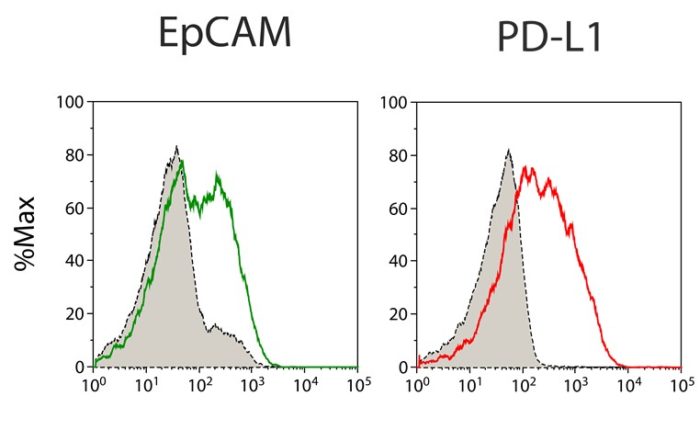
Expression of tumor cell markers and PD-L1 on primary patient-derived cells
Expression of tumor cell markers and PD-L1 on primary material of ovarian cancer patients by flow cytometry. Grey peaks represent the staining with isotype control antibodies. Approaches for Predictive Precision [...]
NTRC and Radboud explore new methods to predict chemotherapy response in ovarian cancer
NTRC and Radboud explore new methods to predict chemotherapy response in ovarian cancer Oss, March, 27th, 2019 – NTRC and Radboudumc Nijmegen (The Netherlands) are developing a platform to predict the response of [...]
Effective discovery of synergistic combinations by computational approaches
In silico approach for the discovery of synergistic combinations Oss, March 19, 2019 - Successful cancer therapies are often based on drug combinations to halt cancer growth and prevent resistance mechanisms. A good way [...]
New reference to Oncolines™ profiling: paper by Rageot et al. in J. Med. Chem.
Rageot et al. (2019) publish on Oncolines™ profiling of PI3K/mTOR inhibitor The phosphoinositide 3-kinase (PI3K) / mechanistic target of rapamycin (mTOR) pathway is frequently overactivated in cancer, and drives cell growth, proliferation, survival, [...]
Tissue-type analysis included in Oncolines™ cancer cell line profiling
Oss, 12 February 2019 – The approval label of many drugs specifically states use in cancer of a certain tissue type, for instance, breast or lung. In any approach to precision medicine, cancer lineage should [...]
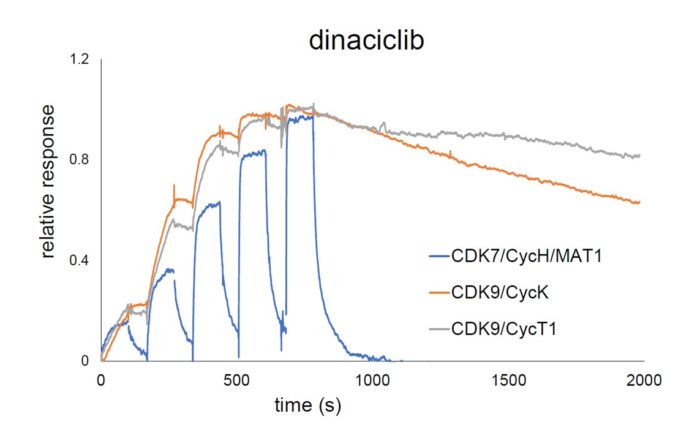
NTRC expands ResidenceTimer™ kinase panel with CDKs, MERTK, TYRO3 and GSKβ
Oss, 20 November 2018 - Analysis of drug binding kinetics is important in predicting the physiological activity and selectivity of drugs. To make such experiments readily accessible on a single platform, NTRC has developed its [...]
NTRC launches the first High-throughput Screening Assay for Arginase-1 inhibitors at ENA2018
Arginase-1 (Arg-1) is an important drug target for cancer immunotherapy. Expression of Arg-1 by tumour-infiltrating myeloid derived suppressor cells induces local L-arginine depletion, which results in reduced T-cell and natural killer cell proliferation. Despite increased [...]
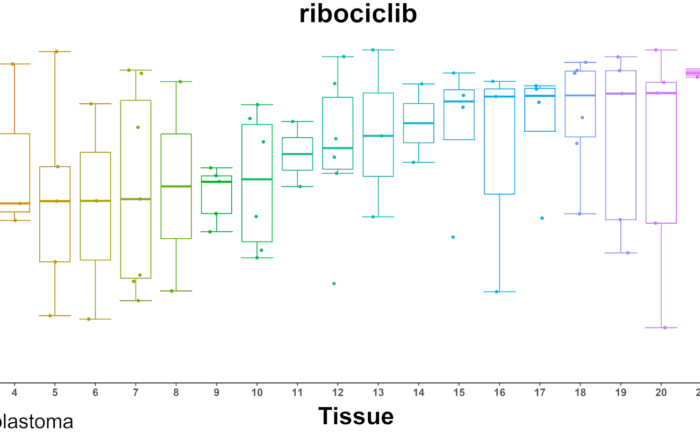
New predictive drug response biomarkers for approved kinase inhibitors
Oss, November, 02, 2018 – Kinase inhibitors illustrate the successful translation of basic scientific knowledge of cancer into new targeted therapies. Selecting the right patient population based on the occurrence of specific cancer driver events [...]
NTRC presents at Rare Diseases and Orphan Drugs conference
Oss, October, 28th, 2018 – At the Rare Diseases and Orphan Drugs Global Forum, held in Belgrade (Serbia) 1st and 2nd November, Dr. Guido Zaman will present on NTRC’s precision medicine platforms for drug discovery [...]
Oncolines™ at Discovery on Target conference in Boston
Oss, September 25th, 2018 – In the Kinase Inhibitor symposium of the Discovery on Target conference, held in Boston this week, Dr. Guido Zaman will present the Oncolines™ profiling of all kinase inhibitors that have [...]
NTRC presents at European Association of Cancer Research conference in Amsterdam
The 25th biannual conference of the European Association of Cancer Research (EACR) with the theme From Fundamental Insight to Rational Cancer Treatment will take place from June 30th to July 2nd in Amsterdam. NTRC will [...]
NTRC expands OncolinesProfiler™ reference compound database to 162 anti-cancer agents
Oss, June, 11th, 2018 – NTRC’s OncolinesProfiler™ service compares inhibitory IC50 signatures of anticancer drugs across 102 cell lines. The signatures belong to a proprietary database generated from the cell line profiling service Oncolines™, which [...]
NTRC presents novel high-throughput screening assay for Arginase I inhibitors for cancer immunotherapy at AACR Annual Meeting in Chicago
NTRC will present two scientific posters at the AACR Annual Meeting 2018, to be held from April 14th to 18th in Chicago. The first poster relates to Arginase, a promising target in the field of [...]
NTRC expands ResidenceTimer™ platform with BTK resistance mutants
Oss, April, 5th, 2018 –NTRC’s ResidenceTimer™ platform enables the determination of the binding kinetics of small molecule kinase inhibitors to their target enzymes. Advantages include: (i) the accurate determination of compound affinity and selectivity; and [...]
NTRC expands SynergyFinder™ platform with triple combination testing
Oss, March, 23rd, 2018 – Many cancer therapies consist of mixtures in which multiple molecular entities work together to halt cancer growth, or to overcome emerging resistance mechanisms. In some cases, as much as five [...]
NTRC includes Growth Rate metric evaluation in Oncolines™ profiling
Oss, February, 22nd, 2018 –Compound profiling in cell line panels is a powerful tool in the development of new cancer medicines. Analysis of drug sensitivity across cell lines can reveal response biomarkers in the form [...]
NTRC at Pivot Oncology conference Breakthroughs in Precision Cancer Treatment
Oss, November, 13th, 2017 – Pivot Park organizes the symposium Breakthroughs in Precision Cancer Treatment on Tuesday November 21st. As part of the conference, NTRC has organized one of the scientific sessions, entitled ‘Drug discovery: [...]
Target residence time-guided lead optimization at the European Pharma Summit in Berlin
Oss, November, 10th, 2017 – At GTC Bio’s European Pharma Summit to be held in Berlin on 16 and 17 November, Dr. Guido Zaman will present on NTRC’s TTK drug discovery program in a scientific [...]
Pharmacogenomic analysis at Molecular Targets and Cancer Therapeutics conference
Oss, October, 16th, 2017 – NTRC will present three scientific posters at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics conference to be held in Philadelphia (USA) from October 27th to 30th. Two of the posters [...]
Novel synergistic combinations identified by unbiased high-throughput screening
Oss, October, 11th, 2017 – A high-throughput screening approach to identify novel synergistic drug combinations has been launched by NTRC under the name SynergyScreen™. The new service product is available as an extension to NTRC’s [...]
NTRC’s key TTK kinase inhibitor patent granted in US
Oss, September, 19th , 2017 – NTRC, a biopharmaceutical company focused on the discovery and development of innovative precision medicines for the treatment of cancer, today announced that the United States Patent and Trademarks Office [...]
Meet NTRC at Discovery on Target conference in Boston 25 – 29 September, 2017
Oss, September, 19th , 2017 – At the 15th Annual Discovery on Target (DOT) conference to be held in Boston (USA) on 25 – 29 September, Dr. Guido Zaman will present on NTRC’s TTK drug [...]
NTRC publishes an association of CTNNB1 mutant status and increased cancer cell line sensitivity to TTK inhibitors
Oss, July, 27th, 2017 - In a new publication, NTRC scientists propose mutations in the cancer hot spot region of the β-catenin gene (CTNNB1) as prognostic drug sensitivity biomarkers for TTK inhibitors. The paper [...]
NTRC expands Oncolines™ cell line panel to 102 cell lines
Oss, June 22nd 2017 – Today NTRC has launched an expansion of its Oncolines™ cancer cell line panel, from 66 to 102 cell lines. Oncolines™ are proliferation assays on a panel of genetically well-characterized cancer [...]
NTRC publishes target residence time-guided optimization of TTK inhibitors
Oss, May 22nd 2017 - This week, NTRC published results from its TTK (Mps1) drug discovery programme in the Journal of Molecular Biology. TTK is a promising new drug target for the treatment of aggressive [...]
NTRC launches GeneNominator™ tool for drug sensitivity and gene expression analysis
Oss, March 30th, 2017 – NTRC announces its GeneNominator™ bioinformatics package to study the biology of inhibitor response at the mRNA expression level. With NTRC’s Oncolines™ panel, the IC50 values of inhibitors can be determined [...]
Meet NTRC at booth #3050 AACR 2017 in Washington D.C.
Oss, March, 27th, 2017 – NTRC will exhibit its services at the AACR conference 2017, to be held in Washington D.C. next week. Please be welcome at booth #3050 in the Exhibition Hall. Two [...]
New study in Oncotarget shows that stable aneuploid cells are more sensitive to TTK inhibition than chromosomally unstable cell lines
Oss, March, 16th, 2017 – In a research article which appeared online today in Oncotarget, NTRC scientists, in collaboration with Prof. Dr. René Medema from the Netherlands Cancer Institute (NKI), show that stable aneuploid tumor [...]
Kinetic parameters of 80 kinase inhibitors available online through the Journal of Molecular Biology
Oss, January, 2nd, 2017 – NTRC has made available the kinetic data of 80 kinase inhibitors on their targets in a research article in the Journal of Molecular Biology. This is the largest data base [...]
Structural insight into the function of TDO in healthy aging
Oss, Groningen, December, 23rd, 2016 – Researchers from the European Research Institute for the Biology of Aging (ERIBA) University of Groningen, the Netherlands) in collaboration with the Netherlands Translational Research Center B.V. have identified an [...]
New drug combinations addressing treatment resistance in childhood leukemia proposed based on SynergyFinder™
Utrecht, Oss, December, 20th, 2016 – Researchers from the Princess Máxima Center for Pediatric Oncology in Utrecht (The Netherlands) in collaboration with researchers from Erasmus Medical Center in Rotterdam have identified mechanisms underlying resistance to [...]
NTRC’s Oncolines™ profiling is cover story in December issue of Molecular Cancer Therapeutics
Oss, December, 5th, 2016 – An analysis by NTRC of 122 anti-cancer agents is cover story of Molecular Cancer Therapeutics, a leading journal on cancer drug discovery from the American Association of Cancer Research (AACR). [...]
NTRC presents on ResidenceTimer™ and OncolinesProfiler™ at 28th EORTC-NCI-AACR symposium in Munich
The 28th EORTC-NCI-AACR symposium on Molecular targets and Cancer therapeutics will take place from November 29th to December 2nd, 2016 in Munich, Germany. NTRC will present two posters: one on the application target residence time [...]
NTRC to join EU mission to Japan and to present at BIO Japan
Oss, September, 13th, 2016 – NTRC will join a mission of European biotechnology companies to Japan, which has been organized by the EU-Japan Centre for Industrial Cooperation. Twenty representatives from eight different European countries, mainly [...]
Comparative Oncolines™ profiling of 122 anti-cancer agents published in Molecular Cancer Therapeutics
Oss, September, 2nd, 2016 – Cancer cell line panels are important tools to determine the activity and selectivity of novel cancer therapeutics, before experiments in animal models and trials in human patients are initiated. In [...]
NTRC presents on ResidenceTimer™ at Discovery on Target conference in Boston
Oss, August, 25th, 2016 – At the Discovery on Target (DOT) conference, to be held in Boston from September 19th to 22nd, Dr. Guido Zaman, Managing Director and Head of Biology at NTRC, will present [...]
NTRC presents OncolinesProfiler™ and TTK program at EACR in Manchester
Oss, July, 4th, 2016 – At the 24th congress of the European Association for Cancer Research (EACR), to be held in Manchester (U.K.) from 9 – 12 July, NTRC will present two posters, one on [...]
Novel synergistic drug combinations proposed for pediatric T-cell leukemia
Oss, Rotterdam, April, 28th, 2016 – Researchers from Erasmus Medical Center and NTRC have discovered novel combinations of experimental small molecule drugs that synergistically block pathologic activation of the IL7-receptor signalling cascade in leukemic cells [...]
NTRC expands number of cancer genes analyzed in Oncolines™ cell line panel
Oss, April 8th, 2016 - Starting from today, NTRC launches a major update of its Oncolines™ bioinformatics platform, referred to as Oncolines™ version 2.0. In the new analysis of cell panel data, 114 new cancer [...]
NTRC presents on TTK kinase program at AACR conference
Oss, April, 8th, 2016 – At the AACR 2016 conference, to be held in New Orleans next week, Jos de Man, Senior Investigator Chemistry at NTRC, will present on the unique binding mode of NTRC’s [...]
NTRC team of cyclists will conquer the Alpe d’Huez to raise funds for cancer research
On Thursday June, 2nd, a team of cyclists from NTRC will participate in Alpe d’HuZes, a fund raising event for cancer research. The aim of the event is to reach the summit of the legendary [...]
NTRC has identified IDO1 inhibitors with best-in-class properties by screening at the European Lead Factory
Oss, December, 9th, 2015 – NTRC today announced that they have identified a series of IDO1 inhibitors with best-in-class properties by screening at the European Lead Factory (ELF). IDO1 is an important target in cancer [...]
NTRC and Vipergen receive Eurostars support for cancer immunotherapy research
Oss, Copenhagen, December 04th, 2015 - NTRC (Netherlands) and Vipergen ApS (Denmark) announced that they have received support from the EUREKA Eurostars Programme for their research on the identification of novel drug candidates for cancer [...]
NTRC launches comparative cancer cell line profiling
Oss, September, 14th, 2015 – NTRC today announced that it has extended its bioinformatics analysis to its Oncolines™ cancer cell line profiling service. OncolinesProfiler™ involves the comparison of a clients’ compound against more than 100 [...]
NTRC presents at Discovery on Target conference in Boston
Guido Zaman and Suzanne van Gerwen will attend the Discovery on Target conference, that will be held in Boston (USA) from September 21st to 24th. On Thursday September 24th at 12.10 p.m., Guido Zaman will [...]
Novel therapeutic target proposed to increase the efficacy of taxane chemotherapy
Oss - Amsterdam, July 10th, 2015 – Researchers from the Netherlands Cancer Institute (NKI), in collaboration with NTRC and University Medical Center Utrecht, have discovered that inhibition of the protein kinase TTK, a protein involved [...]
Whole genome doubling causes multidrug resistance
Oss, July 7th, 2015 – In a new study in Cell Cycle, researchers from the Max Planck Institute and NTRC show that doubling of the whole genome of human cancer cells provides resistance to a [...]
NFK GreenScreen™ and TTK program presented at EACR-AACR conference in Florence
Guido Zaman and Suzanne van Gerwen will attend the EACR-AACR Special Conference, that will be held in Florence (Italy) from June 20th to 23rd. On Monday, June 22nd, two posters will be presented: one on [...]
Selective targeting of cancer driver genes by combinations of existing drugs published in PLOS ONE
Oss, May, 27th, 2015 – NTRC scientists have discovered new combinations of existing drugs to selectively target difficult-to-drug cancer genes, such as MYC and KRAS. The results of these studies have been published today in [...]
TTK drug discovery program presented at Protein Kinases conference in Berlin
Guido Zaman and Suzanne van Gerwen will attend the 10th “Protein Kinases in Drug Discovery” conference, that will be held in Berlin on May 7th and 8th. On Friday, May 8th, Guido Zaman will present [...]
SynergyFinder™ presented at AACR 2015 in Philadelphia
Guido Zaman, Suzanne van Gerwen, Joost Uitdehaag and Jos de Man will attend the Annual Meeting 2015 of the American Association for Cancer Research (AACR), that we be held in Philadelphia (U.S.A.) from April 18th [...]
ResidenceTimer™ and TTK drug discovery program presented at Drug Discovery Chemistry meeting in San Diego
Rogier Buijsman will present and chair one of the sessions of the 6th Kinase Inhibitor Chemistry symposium, which is part of the 10th Annual Drug Discovery Chemistry conference, that will be held in San Diego [...]
NTRC receives Michael J. Fox Foundation support for TDO program for Parkinson’s disease
Oss, April, 3rd, 2015 – NTRC today announced that it has received an award from The Michael J. Fox Foundation For Parkinson’s Research to conduct research on inhibitors of tryptophan-2,3-dioxygenase (TDO), a new treatment option [...]
TTK program and SynergyFinder™ presented at TAT 2015 conference in Paris
NTRC is exhibiting sponsor of the 13th international conference on Targeted Anticancer Therapies (TAT), that we be held in Paris (France) from March 2nd to 4th. NTRC Services will participate in the commercial exhibition and [...]
NTRC receives qualified hit series for TDO from the European Lead Factory
Oss, The Netherlands, 18th February 2015 – NTRC today announced that it has received a collection of fifty small molecule inhibitors for TDO from the European Lead Factory (ELF). TDO (Tryptophan 2,3-dioxygenase) is an important [...]
NTRC launches assay for mouse IDO1 and mouse TDO activity
Cancer immunotherapy aims at the stimulation of the body’s immune system to fight cancer. The T-cell immune response against cancer cells is dampened by the activity of the enzymes indoleamine 2,3-dioxygenase (IDO1) and tryptophan 2,3-dioxygenase [...]
NTRC and Utrecht University start collaborative research project on Parkinson’s disease
Oss, January, 5th, 2014 – Parkinson’s disease is a degenerative disease of the central nervous system. Researchers from NTRC and the group of Dr. Aletta Kraneveld from the Utrecht University Institute of Pharmaceutical Sciences bundle [...]
SynergyFinder™ and NFK GreenScreen™ presented at EORTC-NCI-AACR conference in Barcelona
SynergyFinder™ and NFK GreenScreen™ presented at EORTC-NCI-AACR conference in Barcelona Guido Zaman, Joost Uitdehaag and Suzanne van Gerwen will attend the 26th EORTC-NCI-AACR symposium on “Molecular Targets and Cancer Therapeutics”, that will be held in [...]
NFK GreenScreen™ presented at CHAINS conference in Veldhoven
Nicole Willemsen-Seegers and Joost Uitdehaag will attend the NWO Chemical Sciences conference CHAINS 2014, that will be held in Veldhoven (The Netherlands) on November 17th and 18th. On Monday, November 17th, Joost Uitdehaag will give [...]
NTRC launches Oncolines™ 66 cell line panel
NTRC has extended its Oncolines™ cancer cell line panel to 66 cancer cell lines. The Oncolines™ panel represents a broad range of tumor tissues and all most frequent genetic changes in human cancer. The previous [...]
ResidenceTimer™ will be presented at the EFMC-ISMC symposium 2014
Rogier Buijsman will attend the EFMC-ISMC Medicinal Chemistry symposium, that will be held in Lisbon (Portugal) from September 7th to 11th. Rogier will present a poster on the application of ResidenceTimer™ to identify the target [...]
Reverse Phase Protein Array workshop in Paris sponsored by NTRC
The 4th Global Reverse Phase Protein Array Workshop will take place on October 24-25 at Cité Internationale Universitaire in Paris. The workshop brings together RPPA laboratories, vendors and users to share and discuss results, latest [...]
NTRC will present at 8th International Protein Kinase Inhibitors conference in Warsaw
Guido Zaman and Suzanne van Gerwen will attend and present at the 8th International Conference on Protein Kinase Inhibitors (IPK2014) held in Warsaw, Poland, from September 21st to 25th. The distinguished list of speakers at [...]
Oncolines™ and SynergyFinder™ presented at EACR 2014 in Munich
Guido Zaman and Joost Uitdehaag will attend the Congress of the European Association of Cancer Research (EACR), that will be held in Munich (Germany) from July 5th to 8th. On Monday, July 7th Joost Uitdehaag [...]
NTRC publishes high-throughput screening assay for IDO1 and TDO in Journal of Biomolecular Screening
Immunotherapy is one of the recent advances in cancer treatment. Cancer immunotherapy makes use of the body’s immune system to help fight cancer. The amino acid tryptophan is an important regulator of the immune system [...]
NTRC will present at Discovery Partnerships Conference in Berlin
Guido Zaman will attend IQPC’s Discovery Partnerships Conference held in Berlin on May 19th and 20th. On Tuesday, May 20th, at 4.00 p.m., Guido Zaman will give a presentation on NTRC’s collaborations and drug discovery [...]
NTRC will attend BIO 2014 in San Diego
Gavin Clark, VP Business Development of NTRC, will attend BIO 2014 Conference held in San Diego from June 21st to June 26th. Gavin Clark will be available to meet with interested parties from pharmaceutical industries [...]
NTRC will present at Protein Kinases in Drug Discovery Conference in Berlin
Joost Uitdehaag will attend GTC’s Protein Kinases in Drug Discovery Conference held in Berlin on May 8th and 9th. On Friday, May 9th, at 11.45 p.m., Joost Uitdehaag will give a presentation on the profiling [...]
NTRC launches new service ResidenceTimer™ at Drug Discovery Chemistry conference in San Diego
Rogier Buijsman and Suzanne van Gerwen will attend CHI’s 9th Drug Discovery Chemistry conference held in San Diego from April 23rd to 25th. On Thursday, April 24th, Rogier Buijsman will chair the afternoon session, entitled [...]
Oncolines™ and kinome profiling presented at AACR 2014 in San Diego
Guido Zaman, Jos de Man and Joost Uitdehaag will attend the AACR Annual Meeting 2014, that will be held in San Diego from April 5th to 9th. On Monday, April 7th, from 1:00 to 5:00 [...]
NTRC publishes Oncolines™ profiles of all marketed small molecule kinase inhibitor drugs in PLOS ONE
Oss, March 20th, 2014 – Over the past decade targeted therapies have significantly increased the efficiency of cancer therapy. They bring great benefit to patients because they improve survival rates with much less side effects [...]
NTRC will present at Drug Discovery Innovations Conference 2014
NTRC will present at Drug Discovery Innovations Conference 2014 in Berlin. Date: March 19th, 2014 Session: Cell based assays, phenotypic screening and pioneering technologies www.informa-ls.com/event/DDI2014
NTRC will present at Frontiers in Medicinal Chemistry 2014 in Tübingen.
NTRC will present at the GDCH Frontiers in Medicinal Chemistry 2014 in Tübingen. Date: March 18th, 2014 Session: Inflammation www.gdch.de
NTRC will present at BioEurope Spring 2014
NTRC will present at Bio-Europe Spring 2014 in Turin. Date: March 12, 2014 Time: 11:30 AM Session: Oncology www.ebdgroup.com
NTRC reaches new milestones in their drug discovery programs and expands business development activities
Oss, 6 February 2014 – NTRC B.V. today announced that it has progressed its small molecule kinase inhibitor projects in oncology and immunology to the next phase of pre-clinical development. To support the out-licensing and [...]
NTRC extends the roll-out of its services Oncolines™ and SynergyFinder™ and the kinase profiling services of Carna Biosciences Inc.
NTRC delivers its drug discovery capabilities as fee-for-service for development projects of druggable protein target classes. NTRC is also the European representative for Carna Biosciences Inc. for kinase profiling and reverse phase phospho-protein array technology [...]
NTRC provides high-throughput screening services together with Pivot Park Screening Centre
Oss, November 12th, 2014 – NTRC B.V. and Pivot Park Screening Centre B.V. (PPSC) today announced that they have joined forces in establishing high-throughput screening services. Under the agreement NTRC and PPSC will align their [...]
NTRC to partner with European centers of excellence in training network for next generation cancer researchers
Oss, October 14th, 2014 - Together with nine leading universities and research institutes in Europe and two other companies, Netherlands Translational Research Center B.V. (NTRC) will train eleven young cancer researchers in the field of [...]
NTRC will present at BioEurope 2013
NTRC will present at BioEurope 2013 in Vienna. Date: November 4th-6th, 2013 Time: 9:40 Session: Next Generation www.ebdgroup.com/bioeurope/
Oncolines™ presented at the European Cancer Congress 2013
Joost Uitdehaag will present during the European Cancer Congress 2013. The poster entitled 'Drug sensitivity markers for approved kinase inhibitor drugs from cell panel profiling' decribes the recent results obtained after profiling and bioinformatic analysis [...]
NTRC and Carna join forces in the marketing of drug discovery services in Europe and Japan
Oss - 19 November 2012 - Netherlands Translational Research Center B.V. (NTRC) and Carna Biosciences Inc. (Carna) (Kobe, Japan) announced that they have joined forces in the commercialization and marketing of their drug discovery services [...]
NTRC obtains exclusive license for Merck kinase candidate compounds
Oss - 13 November 2012 - Netherlands Translational Research Center B.V. (NTRC) today announced that it has entered into an exclusive license agreement with Merck & Co, Inc., through its affiliate MSD Oss B.V. Under [...]
NTRC starts at the Life Sciences Park Oss
Oss – 6 June 2012 - Netherlands Translational Research Center B.V. (NTRC) starts operations at the Life Sciences Park Oss, after successful closing its first round of financing. With the financial support of the Brabant [...]