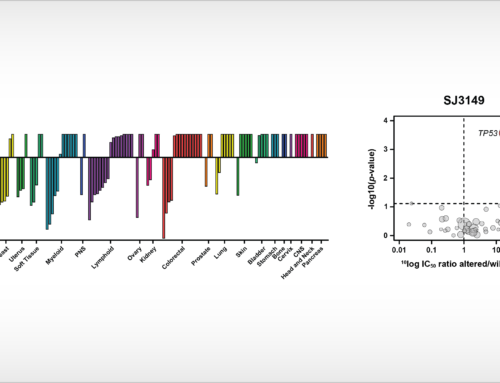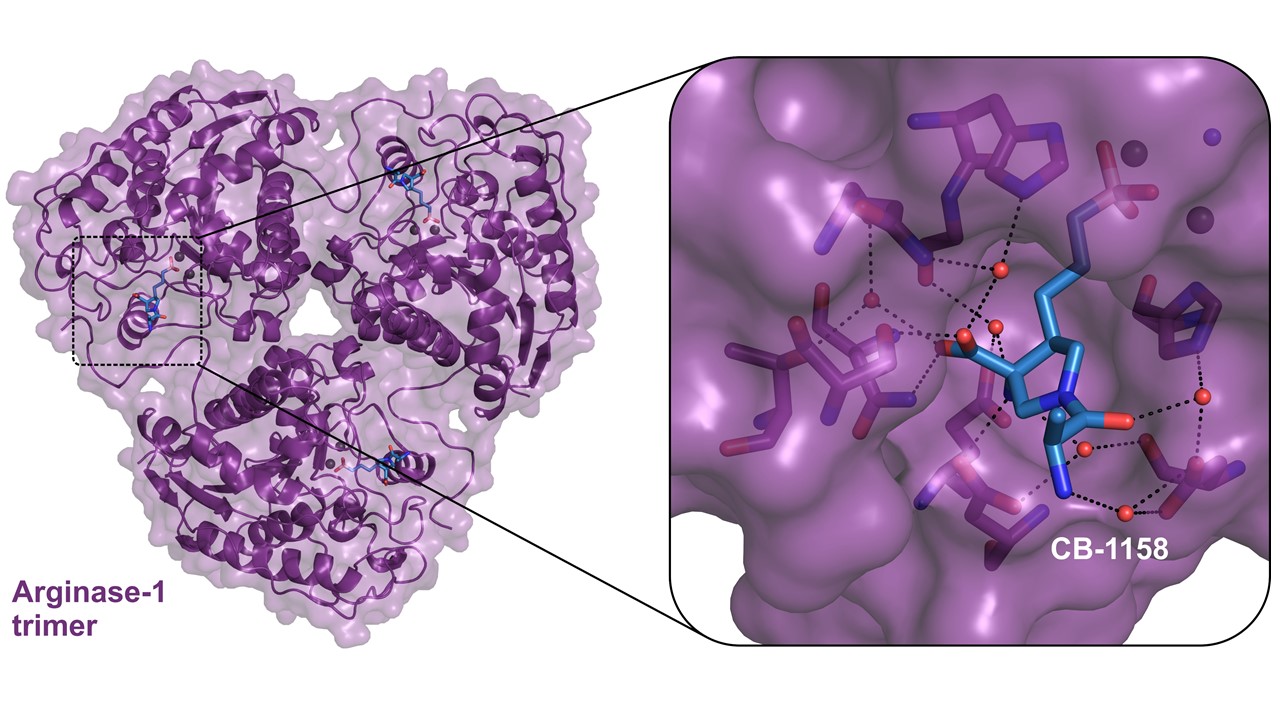
X-ray protein crystal structure of human Arginase-1 with the small molecule inhibitor CB-1158.
New structural insights into Arginase-1, a target for Cancer Immunotherapy
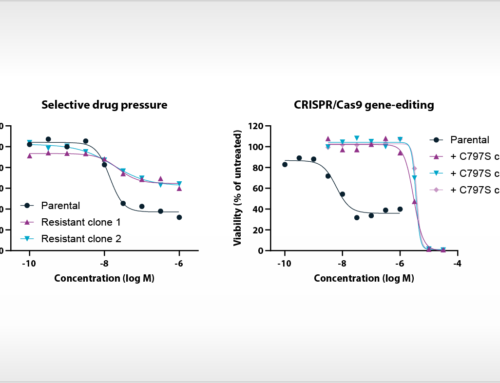
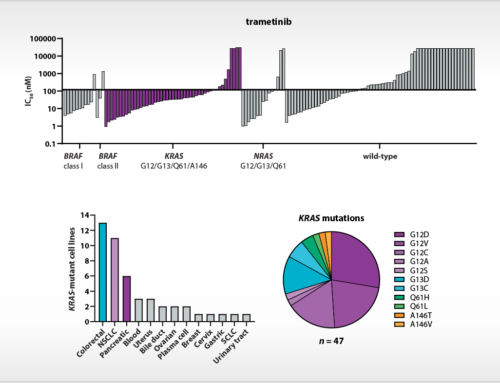
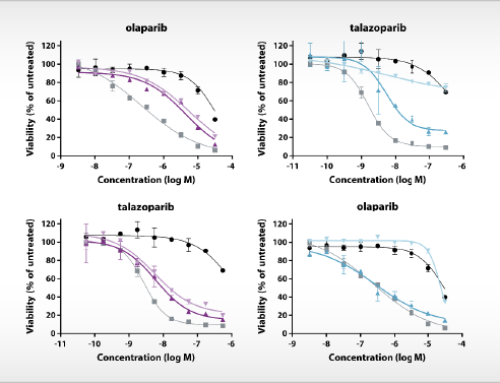
Oss, November, 29th, 2019 – NTRC scientists have published new insights into the atomic basis of inhibition of the cancer immunotherapy target Arginase-1. Their article, which appeared online this week in the Journal of Structural Biology: X (Grobben et al., 2019), discloses the crystal structure of Arginase-1 in complex with the clinical compound CB-1158 (INCB001158) and the reference inhibitor ABH at different pH values. While Arginase-1 has been a drug target for several decades for vascular and pulmonary diseases, it has recently gained new interest due to the discovery of its role in anti-tumour immune response by its release in the tumour microenvironment by tumour-infiltrating myeloid cells (Steggerda et al., 2017). The therapeutic concept that inhibition of Arginase-1 increases the efficacy of the immune checkpoint inhibitor pembrolizumab (Keytruda®) is currently investigated in clinical trials.
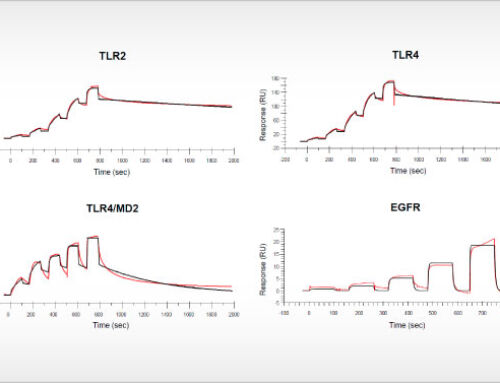
To support new drug discovery on Arginase-1, NTRC scientists resolved the crystal structure of Arginase-1 bound by small molecule inhibitors (Grobben et al., 2019) and developed a new assay method for high-throughput screening (Arginase Gold™). The study in the Journal of Structural Biology: X describes how Arginase-1 adopts slightly different conformations at physiological pH (pH 7.4), and its pH optimum of pH 9.5. Kinetic binding studies via Surface Plasmon Resonance (SPR) show that CB-1158 has slow association kinetics and a long target residence time. The potent enzyme inhibitory activity of the investigational drug CB-1158 in comparison to earlier inhibitors, such as ABH, is explained by its increased rigidity and additional hydrogen-bond interactions with the protein.
The publication in Journal of Structural Biology: X showcases the capabilities of the NTRC team in biochemistry, biophysics and protein X-ray crystallography. NTRC has a hybrid business model, with small molecule drug discovery projects in cancer immunotherapy and Parkinson’s disease. In addition, NTRC provides fee-for-services, by making use of the technology platforms that originally have been developed to support its internal drug discovery projects, such as Oncolines™ and SynergyFinder™.
Table 1. Summary of NTRC’s capabilities in biochemical and biophysical methods.
| Technologies for Biochemical Assays | Orthogonal assays |
| Fluorescence polarization | ELISA |
| Fluorescence intensity | Surface Plasmon Resonance (SPR) |
| Chemiluminescence | Mass-Spectrometry / LC-MS/MS |
| TR-FRET (LANCE®) | Thermal shift |
| AlphaLisa®, AlphaScreen® |
Literature References:
Grobben et al. (2019) Structural insights into human Arginase-1 pH dependence and its inhibition by the small molecule inhibitor CB-1158. Journal of Structural Biology: X, published online, November 26th.
https://doi.org/10.1016/j.yjsbx.2019.100014
Steggerda et al. (2017) Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. Journal for ImmunoTherapy of Cancer, 5: 101.
https://jitc.biomedcentral.com/articles/10.1186/s40425-017-0308-4
NTRC is a precision medicine company dedicated to discovering new anti cancer drug candidates. It is our mission to help you finding a mechanistic hypothesis before entering the clinic. We provide cell-based profiling services, target residence time measurements and biochemical profiling. We can study a wide range of cancer cells, primary patient material and immune cells in vitro, in isolation and in coculture, after exposure to monotherapy and combination therapy. Keywords are: Quality. Flexibility. Short Turnaround Time.