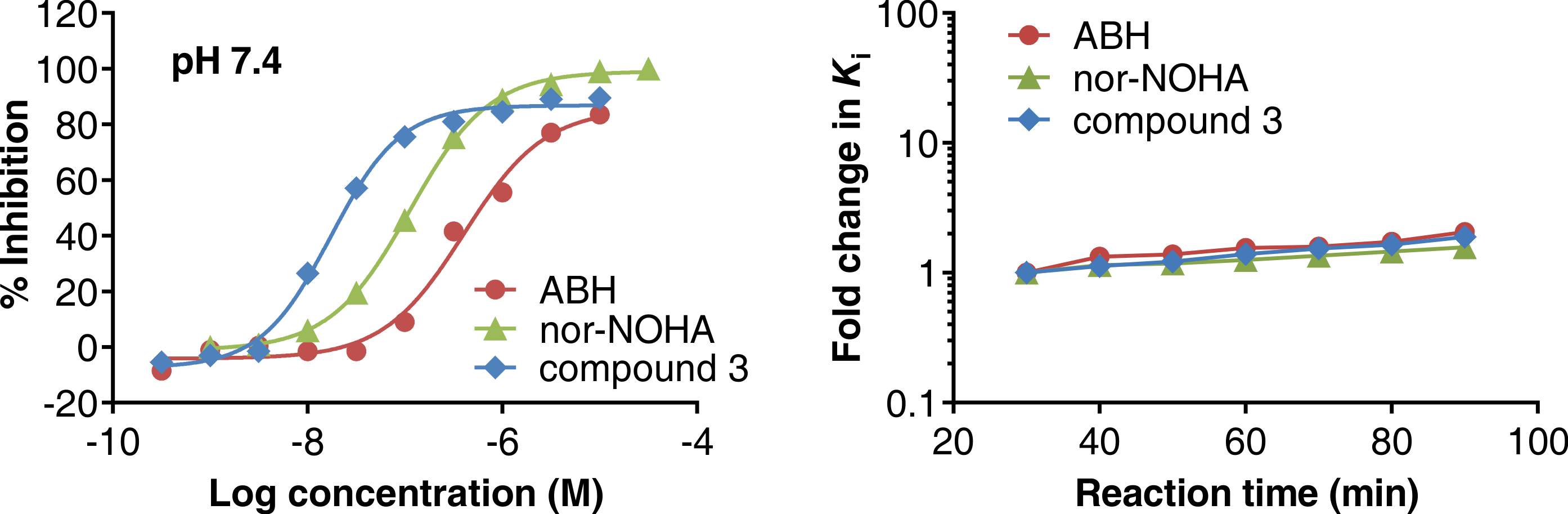
May 18, 2020: Dose response curves of reference inhibitors in the Arginase Gold™ assay (left) and inhibitory constant (Ki) as function of reaction time (right).
Adapted from Grobben et al. (2020).
Screening of Arginase-1 Inhibitors using Arginase Gold™
Oss – May, 18th, 2020 – Arginase-1 is a promising new drug target for cancer immunotherapy. A novel assay technology for high-throughput screening (HTS) of Arginase-1 has been published in SLAS Discovery (Grobben et al., 2020) by researchers from NTRC in collaboration with Pivot Park Screening Centre (PPSC). The assay technology is known as Arginase Gold™.
The enzyme Arginase-1 has a regulatory role in T cell and natural killer cell-mediated immunity in the tumor microenvironment by decreasing levels of the amino acid L-arginine, which is critical for an effective anti-tumor immune response. Preclinical studies have shown that inhibition of Arginase-1 increases tumor immune cell infiltration and decreases tumor growth in model systems for cancer (Steggerda et al., 2017; Czystowska-Kuzmicz et al., 2019; Miret et al., 2019). The first small molecule Arginase-1 inhibitor CB-1158 (INCB001158) is currently being investigated in clinical trials for the treatment of advanced and metastatic tumors as a single agent, and in combination with chemotherapy, immune checkpoint therapy and the IDO1 inhibitor epacadostat (www.clinicaltrials.gov).
Arginase Gold™ is a homogenous (mix-and-measure) enzyme activity assay for Arginase-1, making use of a fluorescent probe developed at NTRC. The assay measures the conversion of L-arginine into its substrate L-ornithine by a decrease in fluorescent signal, thus generating a gain of signal in the presence of inhibitors. In the article, which appeared online today in SLAS Discovery (Grobben et al., 2020), the Arginase Gold™ assay was validated by side-by-side profiling of reference inhibitors in a traditional colorimetric, multi-step urea assay and in the Arginase Gold™ assay. The application in automated HTS was demonstrated by screening a library of small synthetic chemicals at PPSC and deconvolution of active hit compounds. Besides its robustness, easiness to use, and scalability, an appealing characteristic of the Arginase Gold™ assay is that it can be used in a kinetic mode, thus enabling the study of slow-on/slow-off inhibitors, that is, compounds with a long target residence time (Grobben et al., 2020).
Literature references
Grobben et al. (2020) High-throughput fluorescence-based activity assay for Arginase-1, SLAS Discovery, published online first, 15 May 2020.
https://journals.sagepub.com/doi/10.1177/2472555220919340
Steggerda et al. (2017) Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment, Journal for Immunotherapy of Cancer, 5: 101.
https://jitc.bmj.com/content/jitc/5/1/101.full.pdf
Czystowska-Kuzmicz et al. (2019) Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nature Communications, 10: 3000.
https://www.nature.com/articles/s41467-019-10979-3
Miret et al. (2019) Suppression of myeloid cell arginase activity leads to therapeutic response in a NSCLC mouse model by activating anti-tumor immunity, Journal for Immunotherapy of Cancer, 7: 32.
https://jitc.biomedcentral.com/articles/10.1186/s40425-019-0504-5
see www.clinicaltrials.gov for NCT02903914, NCT03314935 and NCT03361228
Grobben et al. (2020) Structural insights into human Arginase-1 pH dependence and its inhibition by the small molecule inhibitor CB-1158, Journal of Structural Biology X, 4: 100014
https://www.sciencedirect.com/science/article/pii/S2590152419300121?via%3Dihub
NTRC is a precision medicine company dedicated to the development of new anti-cancer drugs. NTRC facilitates the development of novel therapies by providing custom-based assays with primary patient material and high-throughput cancer cell line profiling services (Oncolines™, GeneNominator™, SynergyFinder™ and SynergyScreen™), as well as target residence time measurements for protein kinases (ResidenceTimer™) on a fee-for-service basis. Arginase Gold™ and NFK GreenScreen™ are assay read-outs for the cancer immunotherapy drug targets Arginase-1, and IDO1 and TDO respectively. Assay kits are supplied to clients globally.